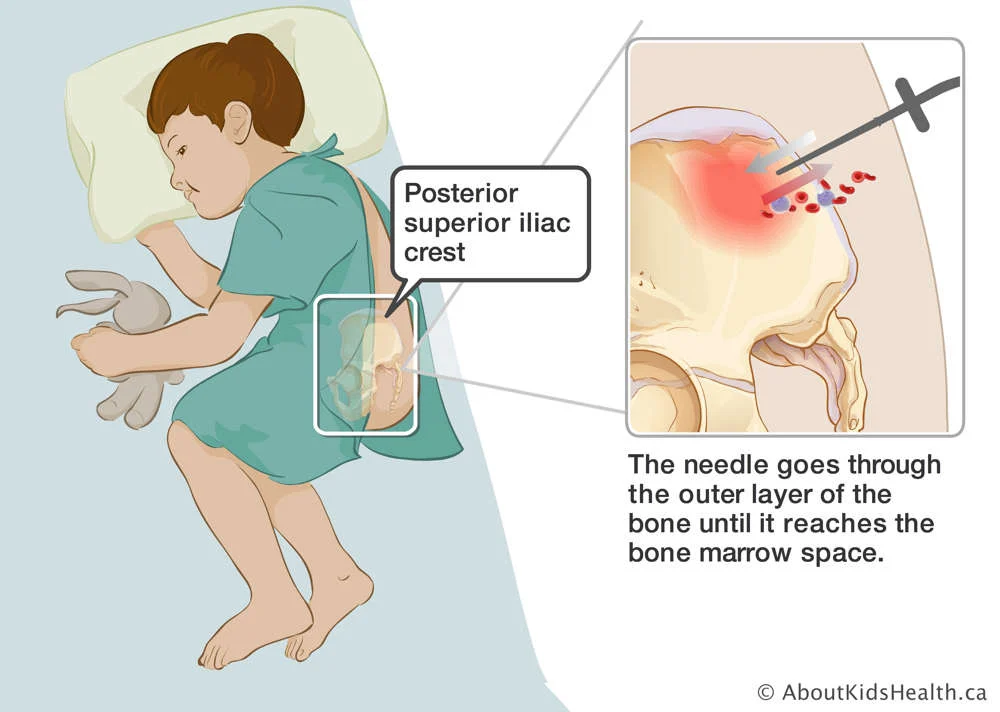
Cellular starting materials are the foundational biological components used to initiate the manufacturing process for cell-based therapies. These materials consist of living cells collected from patients (autologous) or donors (allogeneic), serving as the raw input for isolation, expansion, genetic modification, and formulation into advanced therapy medicinal products (ATMPs).
The quality, consistency, and characteristics of starting materials critically influence the safety, potency, and efficacy of the final cell therapy product. Regulatory bodies like the FDA and EMA classify them under Good Manufacturing Practice (GMP) guidelines, requiring rigorous donor screening, collection, testing, and traceability. With the cell therapy market surging, optimizing starting materials is key to scalability, cost reduction, and broader accessibility.
Importance in Cell Therapy Manufacturing
The manufacturing workflow begins with starting materials and proceeds through isolation/selection, culture/expansion, modification (if needed), formulation, and release testing.
Variability in starting materials (e.g., cell count, viability, patient health) poses major challenges for autologous therapies, often leading to manufacturing failures. Allogeneic approaches aim for standardization via master cell banks.
Types of Cellular Starting Materials
1. Peripheral Blood-Derived (Leukopaks/Leukapheresis Products)
The most common for immune cell therapies like CAR-T. Apheresis collects mononuclear cells (T cells, NK cells) from mobilized or non-mobilized blood.
Used in approved CAR-T products (e.g., Kymriah, Yescarta).
2. Bone Marrow Aspirate
Source of hematopoietic stem cells (HSCs) for transplants and some gene therapies.
Traditional for HSCT; less common now due to peripheral mobilization.
3. Induced Pluripotent Stem Cells (iPSCs)
Reprogrammed adult cells (e.g., skin fibroblasts, blood) into pluripotent state, then differentiated into desired lineages (neurons, cardiomyocytes).
Ideal for allogeneic “off-the-shelf” therapies.
4. Tumor Tissue (for TILs)
Resected tumors digested to isolate tumor-infiltrating lymphocytes for solid tumor therapies (e.g., Lifileucel for melanoma).
5. Other Sources
Umbilical cord blood, adipose tissue (for MSCs), or specific tissues (e.g., skin for keratinocytes).
Autologous vs. Allogeneic Starting Materials
- Autologous — Patient’s own cells; no rejection risk but high variability, long vein-to-vein time, expensive.
- Allogeneic — Donor or engineered universal cells; scalable, consistent, immediate availability but risk of GVHD or immune rejection (mitigated by editing).
Shift toward allogeneic for commercialization.
Collection, Processing, and Quality Considerations
Collection occurs in GMP-compliant facilities with strict donor eligibility (infectious disease testing, health history).
Chain of custody/identity essential. Critical quality attributes: Viability (>70–90%), purity, potency markers (e.g., CD3+ for T cells).
Challenges: Apheresis variability, low yields in diseased patients, cryopreservation effects.
Regulatory and Future Trends
FDA/EMA require starting materials to be “well-characterized” with comparability studies for changes. Emerging: Healthy donor banks, automated apheresis, gene-edited universal cells (e.g., hypoimmune iPSCs), in vivo sourcing.
In conclusion, cellular starting materials are the bedrock of cell therapy success. Advances in collection standardization, allogeneic sources, and automation are addressing variability and scalability, paving the way for more accessible curative treatments. For specific therapies, refer to guidelines from ISCT or regulatory agencies.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/carbon-disulfide-market
https://www.zionmarketresearch.com/de/report/fish-pumps-market
https://www.zionmarketresearch.com/de/report/electric-arc-furnace-eaf-dust-recycling-market
https://www.zionmarketresearch.com/de/report/early-childhood-education-market
https://www.zionmarketresearch.com/de/report/desiccated-coconut-powder-market-size