Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator gas administered as a medical therapy to improve oxygenation and reduce pulmonary vascular resistance in specific clinical conditions. Delivered via mechanical ventilation or specialized devices, iNO acts locally in the lungs with minimal systemic effects due to rapid inactivation by hemoglobin. It is colorless, odorless, and highly reactive, requiring precise dosing and monitoring.
First discovered as endothelium-derived relaxing factor (EDRF) in the 1980s (identified as NO by Robert Furchgott, Louis Ignarro, and Ferid Murad—Nobel Prize 1998), therapeutic iNO was pioneered in the early 1990s for neonatal pulmonary hypertension. FDA approval came in 1999 for persistent pulmonary hypertension of the newborn (PPHN). As of 2025, iNO remains a standard treatment in neonatology, with expanding off-label use in pediatrics, adults, and perioperative settings. The Global Medical INO Market is valued at approximately USD 800 million-1 billion, dominated by systems from Mallinckrodt (INOmax), Linde, and Praxair, though high cost (~USD 3,000-5,000/day) limits broader adoption.
iNO exemplifies targeted gas therapy, revolutionizing management of hypoxic respiratory failure while highlighting challenges in cost, delivery, and evidence for expanded indications.

Physiological Mechanism
Nitric oxide is an endogenous signaling molecule:
- Produced by nitric oxide synthase (NOS) from L-arginine.
- Diffuses into vascular smooth muscle.
- Activates soluble guanylate cyclase → increased cGMP → smooth muscle relaxation → vasodilation.
Inhaled NO:
- Diffuses selectively into ventilated alveoli.
- Dilates pulmonary vessels in well-ventilated regions (improves V/Q matching).
- Rapidly binds hemoglobin (forming methemoglobin and nitrosylhemoglobin), preventing systemic vasodilation.
Key effects:
- Reduces pulmonary artery pressure (PAP).
- Improves oxygenation (PaO₂/FiO₂ ratio).
- Minimal systemic hypotension.
Metabolism: Converted to nitrate/nitrite; excreted renally.
Approved Indications
- Persistent Pulmonary Hypertension of the Newborn (PPHN) FDA/EMA-approved: Term/near-term neonates (>34 weeks) with hypoxic respiratory failure and echocardiographic evidence of pulmonary hypertension.
- Starting dose: 20 ppm.
- Duration: Until oxygenation improves (days-weeks).
- Perioperative Use in Congenital Heart Disease Approved in some regions for pulmonary hypertension post-cardiac surgery.
Off-Label and Investigational Uses
- Acute Respiratory Distress Syndrome (ARDS) Improves oxygenation short-term; no mortality benefit (meta-analyses).
- Pediatric/Adult Pulmonary Hypertension Acute vasoreactivity testing; chronic use limited by rebound.
- Bronchopulmonary Dysplasia (BPD) Prevention/treatment in preterm infants (mixed results).
- Sickle Cell Disease Acute chest syndrome.
- Cardiogenic Shock/Post-Cardiac Surgery Right ventricular support.
- COVID-19/ARDS Temporary oxygenation improvement; no survival benefit.
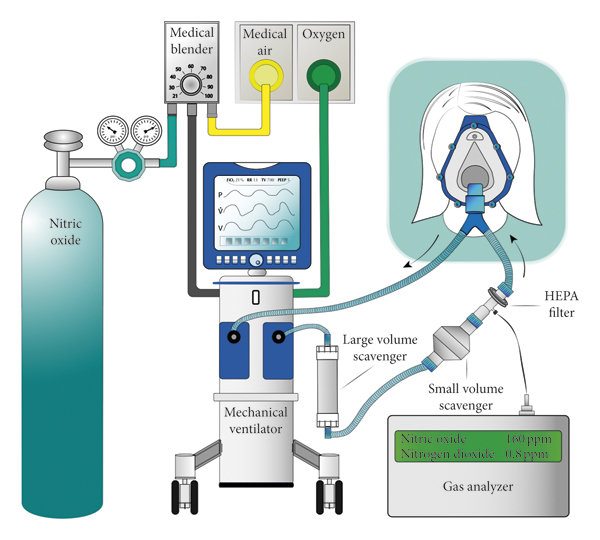
Delivery Systems
iNO requires specialized equipment:
- INOmax DSIR/INOvent: FDA-approved integrated systems.
- Cylinder Gas: 800-1000 ppm NO in nitrogen; blended with ventilator flow.
- Monitoring: Continuous NO, NO₂ (toxic byproduct), methemoglobin.
- Scavenging: Exhaust systems prevent environmental exposure.
Dosing: 5-20 ppm (higher ineffective/toxic). Weaning gradual to avoid rebound hypertension.
Clinical Evidence
- PPHN: Pivotal trials (NINOS, 1997; Davidson, 1998) showed reduced ECMO need without mortality difference.
- ARDS: Large RCTs (meta-analysis 2020s) confirm transient oxygenation benefit, no survival gain.
- Preterm Infants: Early enthusiasm tempered; no neurodevelopmental benefit, possible harm.
Guidelines:
- ATS/ESICM/SCCM: Not recommended routinely for ARDS.
- Neonatal: Standard for PPHN with echocardiography.
Safety and Side Effects
iNO is generally safe at approved doses:
- Methemoglobinemia: >2-3% levels; reversible.
- NO₂ Toxicity: Lung injury if >3-5 ppm.
- Rebound Pulmonary Hypertension: Abrupt discontinuation.
- Bleeding Risk: Theoretical platelet inhibition (minimal clinical).
Contraindications:
- Left ventricular dysfunction (worsens pulmonary edema).
- Methemoglobin reductase deficiency.
Monitoring: MetHb, NO/NO₂ levels mandatory.
Cost and Access
High cost:
- USD 100-150/hour (gas + delivery system).
- Limits use in low-resource settings.
Alternatives: Sildenafil (oral/IV), prostacyclin analogs.
Future Directions
- Portable/ambulatory devices.
- Combination with other vasodilators.
- Inhaled NO donors (organic nitrates).
- Extracorporeal NO removal for toxicity.
Conclusion
Inhaled nitric oxide remains a life-saving therapy for neonatal PPHN and select perioperative cases, offering targeted pulmonary vasodilation with a favorable safety profile at proper doses. Despite transient benefits in broader hypoxic conditions, lack of mortality improvement limits routine adult use. Cost and delivery complexity constrain access, but ongoing research into alternatives and portable systems may expand applications. iNO exemplifies precision gas medicine, balancing potent effects with careful monitoring in critical care.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/car-air-freshener-market
https://www.zionmarketresearch.com/de/report/unmanned-combat-aerial-vehicle-market
https://www.zionmarketresearch.com/de/report/sulphur-and-sulfuric-acid-market
https://www.zionmarketresearch.com/de/report/organic-yeast-market
https://www.zionmarketresearch.com/de/report/linear-shower-drains-market