Metformin hydrochloride is the most widely prescribed oral antidiabetic medication worldwide, belonging to the biguanide class. It is the first-line pharmacological treatment for type 2 diabetes mellitus (T2DM) and is also used off-label for conditions such as polycystic ovary syndrome (PCOS), prediabetes, and metabolic syndrome. Chemically, it is 1,1-dimethylbiguanide hydrochloride (C₄H₁₁N₅·HCl), a white crystalline powder with high water solubility.
Derived from the French lilac (Galega officinalis), a plant historically used for diabetes symptoms, the active compound guanidine was isolated in the 1920s. Metformin was synthesized in the 1920s but gained clinical use in the late 1950s (France) and 1995 (U.S. FDA approval as Glucophage). As of 2025, metformin remains on the WHO List of Essential Medicines, with generic formulations dominating. Global consumption exceeds billions of tablets annually, valued at USD 4-6 billion in the antidiabetic market segment. Immediate-release (IR), extended-release (XR), and combination products (e.g., with DPP-4 inhibitors, SGLT2 inhibitors) are available.
Metformin is renowned for efficacy, safety, low cost, and potential cardiovascular/anti-aging benefits, though gastrointestinal tolerance and rare lactic acidosis risk require consideration.
Chemical Structure and Properties
Metformin hydrochloride:
- Molecular weight: 165.63 g/mol.
- Appearance: White, odorless crystals.
- Solubility: Highly soluble in water (>300 mg/mL).
- pKa: 12.4 (strongly basic).
- Stability: Hygroscopic; stable in solid form.
Pharmacokinetics:
- Absorption: Oral bioavailability ~50-60%; saturation at higher doses.
- Distribution: Minimal protein binding; volume ~650 L.
- Metabolism: None (not hepatically metabolized).
- Excretion: Renal (unchanged); half-life 4-8 hours.
- Accumulation Risk: In renal impairment.
Mechanism of Action
Metformin’s primary effects are glucose-lowering without hypoglycemia risk:
- Hepatic Glucose Production Suppression
- Inhibits gluconeogenesis/glycogenolysis via AMPK activation (energy sensor).
- Reduces mitochondrial complex I activity → altered ATP/AMP ratio.
- Improved Insulin Sensitivity
- Enhances peripheral glucose uptake (muscle, adipose).
- Gut Effects
- Delays glucose absorption.
- Alters microbiome (increases Akkermansia).
- GLP-1 secretion increase (minor).
No direct pancreatic beta-cell stimulation → low hypoglycemia risk.
Additional pleiotropic effects:
- Weight-neutral/loss.
- Lipid profile improvement.
- Anti-inflammatory.
- Potential anti-cancer/anti-aging (mTOR inhibition, AMPK).
Clinical Indications
Approved:
- Type 2 Diabetes Mellitus (monotherapy or combination).
- Prediabetes (off-label in some guidelines).
Off-Label/Investigational:
- PCOS: Improves ovulation, insulin resistance.
- Gestational Diabetes: Alternative to insulin.
- Weight Management: Metabolic syndrome.
- Anti-Aging/Longevity: TAME trial ongoing (2025 status: recruitment phase).
Guidelines (ADA/EASD 2025):
- First-line for T2DM (all patients unless contraindicated).
- Preferred with CVD, heart failure, CKD (renal/cardioprotective).
Dosage and Administration
- Starting: 500 mg once/twice daily with meals (reduce GI effects).
- Titration: Increase 500 mg weekly; max 2,000-2,550 mg/day.
- XR Formulation: Once-daily; better tolerance.
- Renal Adjustment: eGFR >45 mL/min full dose; 30-45 cautious; <30 contraindicated.
Combinations: Metformin + SGLT2i, GLP-1RA, DPP-4i, sulfonylurea.
Efficacy
- HbA1c Reduction: 1-2% (monotherapy).
- Weight: Neutral to -2-3 kg.
- Cardiovascular: UKPDS legacy effect; reduced MI/mortality in overweight T2DM.
- Renal: REDUCE-IT subgroup benefits.
Long-term: Delays T2DM onset in prediabetes (DPP study).
Safety Profile and Side Effects
Excellent overall:
- Common (>10%): GI (nausea, diarrhea, bloating)—dose-related, transient; XR better tolerated.
- Vitamin B12 Deficiency: 10-30% long-term (malabsorption); monitor/supplement.
- Lactic Acidosis: Very rare (~3-10/100,000 patient-years); almost exclusively with contraindications (renal failure, hypoxia).
Contraindications:
- eGFR <30 mL/min.
- Acute/severe heart failure, shock.
- Severe liver disease, alcoholism.
- IV iodinated contrast (temporary hold).
Drug Interactions
- Minimal CYP metabolism → few pharmacokinetic.
- Cationic drugs (e.g., cimetidine) compete renal excretion.
- Alcohol increases lactic acidosis risk.
Special Populations
- Pregnancy: Category B; used in GDM/PCOS.
- Elderly: Renal function monitoring.
- Children: Approved ≥10 years T2DM.
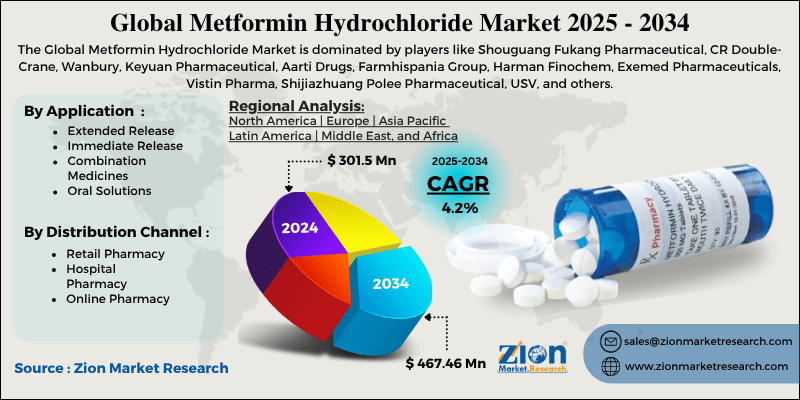
Market and Formulations
Generic dominant; brands: Glucophage, Fortamet (XR), Riomet (liquid). Combinations: Janumet (sitagliptin), Xigduo (dapagliflozin).
Trends: Bioequivalent generics, delayed-release (Gut-specific).
Emerging Research
- Cardioprotection: Beyond glucose (AMPK, inflammation).
- Anti-Aging: TAME trial targets age-related diseases.
- Cancer: Reduced incidence/progression (observational).
- Neuroprotection: Parkinson’s, Alzheimer’s preclinical.
Conclusion
Metformin hydrochloride remains the cornerstone of type 2 diabetes management over 65 years after introduction, offering glucose control, cardiovascular benefits, and excellent safety at low cost. Its pleiotropic effects extend to PCOS, prediabetes, and potential longevity applications. GI tolerance and B12 monitoring are key considerations. As evidence evolves, metformin continues exemplifying effective, accessible pharmacotherapy in metabolic disease.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/epoxy-composite-market
https://www.zionmarketresearch.com/de/report/packaged-food-essence-ingredient-market
https://www.zionmarketresearch.com/de/report/fashion-event-market
https://www.zionmarketresearch.com/de/report/secondary-macronutrients-market
https://www.zionmarketresearch.com/de/report/refurbished-smart-watches-market