Constrained Peptide Drugs represent an emerging class of therapeutics that bridge the gap between small-molecule drugs and biologics like antibodies. These molecules are peptides engineered to adopt rigid, predefined conformations through chemical modifications, enhancing their stability, selectivity, and pharmacokinetic properties. Unlike linear peptides, which are prone to degradation and poor bioavailability, constrained peptides are “locked” into bioactive shapes, making them ideal for targeting challenging interactions such as protein-protein interfaces (PPIs).
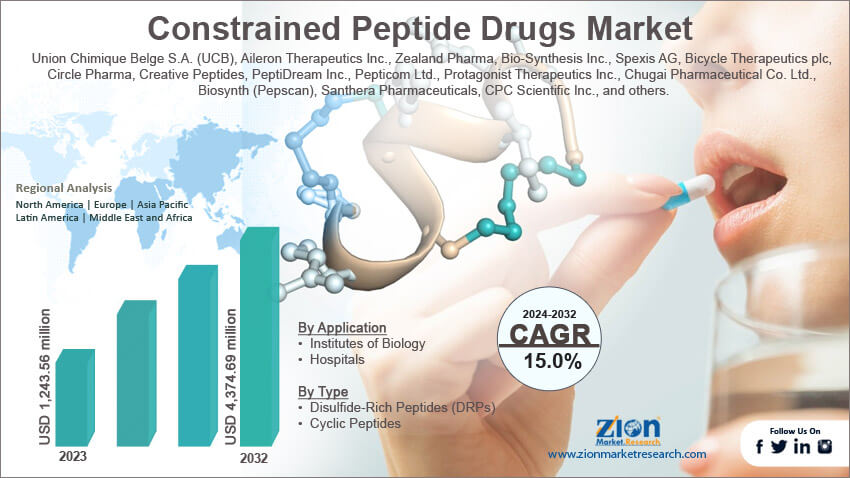
The concept dates back to the 1980s with early cyclization techniques, but advancements in synthesis and design have propelled them into clinical pipelines since the 2010s. By 2025, the constrained peptide drugs market is projected to grow significantly, driven by their potential in oncology, immunology, and metabolic disorders, with estimates valuing it at several billion USD globally. Key players include companies like Protagonist Therapeutics and Bicycle Therapeutics, focusing on oral bioavailability and precision targeting. These drugs offer advantages like high specificity, low toxicity, and manufacturability, positioning them as a promising alternative in the post-antibody era.
Chemical and Physical Properties
Constrained peptides are typically 5-40 amino acids long, with molecular weights ranging from 500-5,000 Da, placing them between small molecules and proteins. Their defining feature is conformational rigidity, achieved through covalent bonds that restrict flexibility, mimicking stable secondary structures like α-helices or β-turns. Common constraints include disulfide bridges, lactam bridges, hydrocarbon staples, or macrocyclization via click chemistry. Physically, they exhibit improved resistance to proteolysis (half-lives extended from minutes to hours or days), enhanced membrane permeability, and better binding affinities (often in the nanomolar range). Solubility varies by sequence and modification; hydrophilic staples can improve aqueous solubility, while hydrophobic ones enhance cell penetration. Thermodynamic stability is high, with melting points often above 100°C for cyclic forms. Spectroscopic properties, such as circular dichroism, confirm constrained helicity or turns.
Production Methods
Synthesis of constrained peptides combines Solid-Phase Peptide Synthesis (SPPS) with post-synthetic modifications. SPPS, pioneered by Merrifield, assembles linear chains on resin supports using Fmoc or Boc chemistry. Constraint introduction follows: for cyclization, orthogonal protecting groups enable selective deprotection and coupling (e.g., head-to-tail amide bonds). Hydrocarbon stapling uses olefin metathesis with non-natural amino acids like α-methyl-α-alkenyl residues. Enzymatic methods, such as sortase-mediated ligation, offer bioorthogonal alternatives for larger peptides. Purification via HPLC ensures >95% purity, with mass spectrometry confirming structure. Scalable production reaches gram quantities for clinical trials, with costs decreasing through automation. Innovations include mRNA display for library screening and AI-driven design to predict optimal constraints.
Applications and Uses
Constrained peptides excel in modulating PPIs, a challenge for traditional drugs. In oncology, they target MDM2-p53 (e.g., ALRN-6924 in trials) or BCL-2 family proteins for apoptosis induction. Immunological applications include IL-17 inhibitors for autoimmune diseases. Metabolic disorders benefit from GLP-1 mimetics with enhanced half-lives. Other uses: Antimicrobial peptides against resistant bacteria, and CNS therapeutics crossing the blood-brain barrier. Delivery forms include oral (via permeability enhancers), injectable, or topical. Bicycle peptides, a subclass, use tandem cyclization for bivalent targeting.
Health and Safety Considerations
Constrained peptides generally exhibit low immunogenicity and toxicity due to their small size and human-like sequences. Preclinical studies assess stability in plasma, off-target effects, and pharmacokinetics. Potential risks include injection-site reactions or renal accumulation for larger variants. Clinical trials monitor for hypersensitivity, with most candidates showing favorable safety profiles. Compared to antibodies, they offer faster clearance and reduced Fc-mediated effects.
Environmental Impact
Peptide synthesis is resource-intensive, involving solvents and resins, but green chemistry advancements (e.g., water-based SPPS) mitigate this. Biodegradability is high, reducing bioaccumulation. Manufacturing scales aim for sustainability through recycling and biocatalysis.
Regulations and Recommendations
FDA classifies them as biologics or small molecules based on size; IND processes require detailed CMC data. EMA guidelines emphasize conformational characterization. Recommendations: Prioritize oral bioavailability research and PPI-focused libraries for future development. Ongoing trials and approvals will solidify their role in precision medicine.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/neopentyl-glycol-npg-market
https://www.zionmarketresearch.com/de/report/food-minerals-market
https://www.zionmarketresearch.com/de/report/optical-lens-for-infrared-device-market
https://www.zionmarketresearch.com/de/report/communications-service-providers-csp-market
https://www.zionmarketresearch.com/de/report/animal-radio-collar-market