Omics-Based Clinical Trials integrate high-throughput technologies—genomics, transcriptomics, proteomics, metabolomics, epigenomics, and increasingly multi-omics—to stratify patients, identify biomarkers, predict treatment responses, and guide precision medicine.
These trials shift from one-size-fits-all approaches to molecularly informed designs, improving efficacy, reducing adverse events, and accelerating drug development. As of 2025, the global omics-based clinical trials market is valued at approximately USD 33–35 billion, projected to reach USD 53–70 billion by 2030–2034 with a CAGR of 7–8%, driven by precision oncology, regulatory support, and AI integration.
History and Evolution
Omics technologies emerged in the post-Human Genome Project era (2003), with early applications in biomarker discovery. The Institute of Medicine’s 2012 report Evolution of Translational Omics established rigorous criteria for omics-based tests in trials, addressing premature uses (e.g., Duke University cases).
Regulatory milestones include FDA/EMA support for biomarker-driven designs (e.g., PREA/BPCA in pediatrics, EU Paediatric Regulation). The 2010s saw basket/umbrella trials (e.g., NCI-MATCH, 2015). By the 2020s–2025, multi-omics and AI integration accelerated, with approvals like companion diagnostics for targeted therapies.
Principles and Workflow
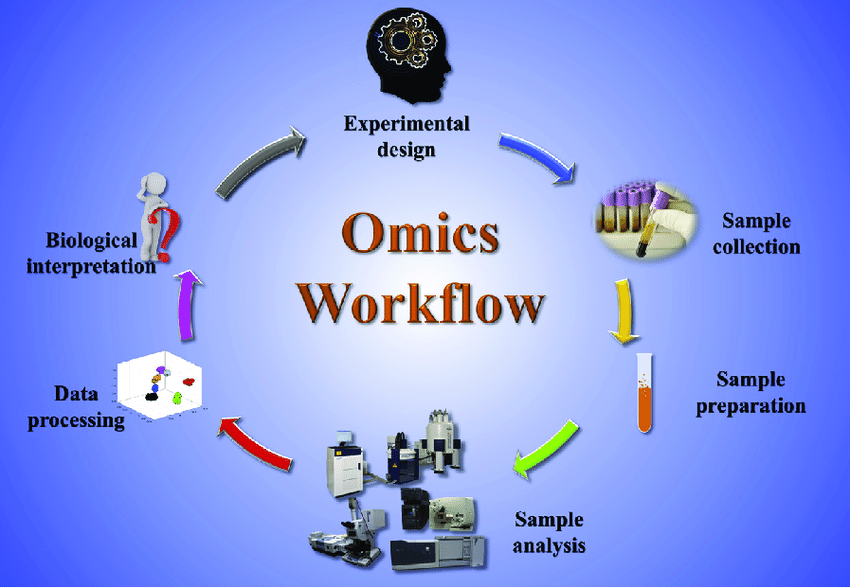
Omics-Based Trials profile molecular layers:
- Genomics: DNA mutations, CNVs.
- Transcriptomics: RNA expression.
- Proteomics: Protein abundance/modifications.
- Metabolomics/Epigenomics: Metabolites, methylation.
Multi-omics integrates these for holistic views.
Workflow: Sample collection → High-throughput assay → Data analysis (AI/ML) → Biomarker validation → Patient stratification.
Trial Designs
- Basket Trials — Test one drug across multiple cancers sharing a biomarker.
- Umbrella Trials — Multiple drugs for one cancer type, stratified by biomarkers.
- Platform/Adaptive Trials — Dynamic arms based on real-time omics data.
Applications
Primarily oncology (46% market share in 2024), with basket trials for rare mutations. Expanding to autoimmune, neurology, and rare diseases. Examples: NCI-MATCH, Tumor Profiler (multi-omics for melanoma).
Advantages
- Improved patient selection → Higher response rates.
- Biomarker discovery → Companion diagnostics.
- Reduced costs/failures → Early-phase integration.
- Real-world evidence → Liquid biopsies, ctDNA monitoring.
Challenges
- Data complexity → Integration, standardization.
- High costs → Sequencing, analytics.
- Ethical/regulatory → Privacy, validation rigor.
- Variability → Reproducibility across platforms.
Current Trends (2025)
- AI/ML for multi-omics → Predictive models, digital twins.
- Spatial/single-cell omics → Tumor heterogeneity.
- Federated learning → Privacy-preserving collaboration.
- Growth in Phase I/III → Interventional designs dominate.
In conclusion, omics-based clinical trials are revolutionizing precision medicine, particularly in oncology, by enabling molecularly guided therapies. Advances in AI, multi-omics integration, and adaptive designs promise higher success rates and personalized care. For ongoing trials, consult databases like ClinicalTrials.gov or regulatory guidelines from FDA/EMA.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/chinese-hamster-ovary-cells-cho-market
https://www.zionmarketresearch.com/de/report/vegetable-hpmc-capsule-market
https://www.zionmarketresearch.com/de/report/carpet-cleaning-machine-market
https://www.zionmarketresearch.com/de/report/ldpe-decking-market
https://www.zionmarketresearch.com/de/report/grow-tents-market